Metals have a characteristic lustrous appearance, often described as shiny or metallic. Metals are typically malleable, meaning they can be easily hammered, pressed, or rolled into various shapes without breaking. Metals can vary in their reactivity. They are applied to many important areas of our lives as well as tools in specific industries. Today, let’s learn about 45 of the heaviest metals created in the lab or found on Earth.
1. Oganesson (Og)

Oganesson
| Name, Symbol | Oganesson, Og |
| Atomic number | 118 |
| standard atomic mass | [294] |
Oganesson (Og) is a highly radioactive synthetic element with the atomic number 118 and the symbol Og. It is predicted to have properties similar to other noble gasses like helium, neon, argon, krypton, xenon, and radon. It is likely to be a colorless and odorless gas at room temperature. As a superheavy and highly unstable element, Oganesson’s isotopes have extremely short half-lives.
2. Tennessine (Ts)

| Name, Symbol | Tennessine, Ts |
| Atomic number | 117 |
| standard atomic mass | [294] |
As a synthetic element, tennessine is not found naturally on Earth and can only be produced in a laboratory through nuclear reactions. Its most stable isotope, tennessine-294, has a very short half-life of about 50 milliseconds. It is likely to be a highly reactive element, readily forming compounds with other elements. Further research and experimentation are required to fully understand the properties of tennessine and its potential applications.
3. Livermorium
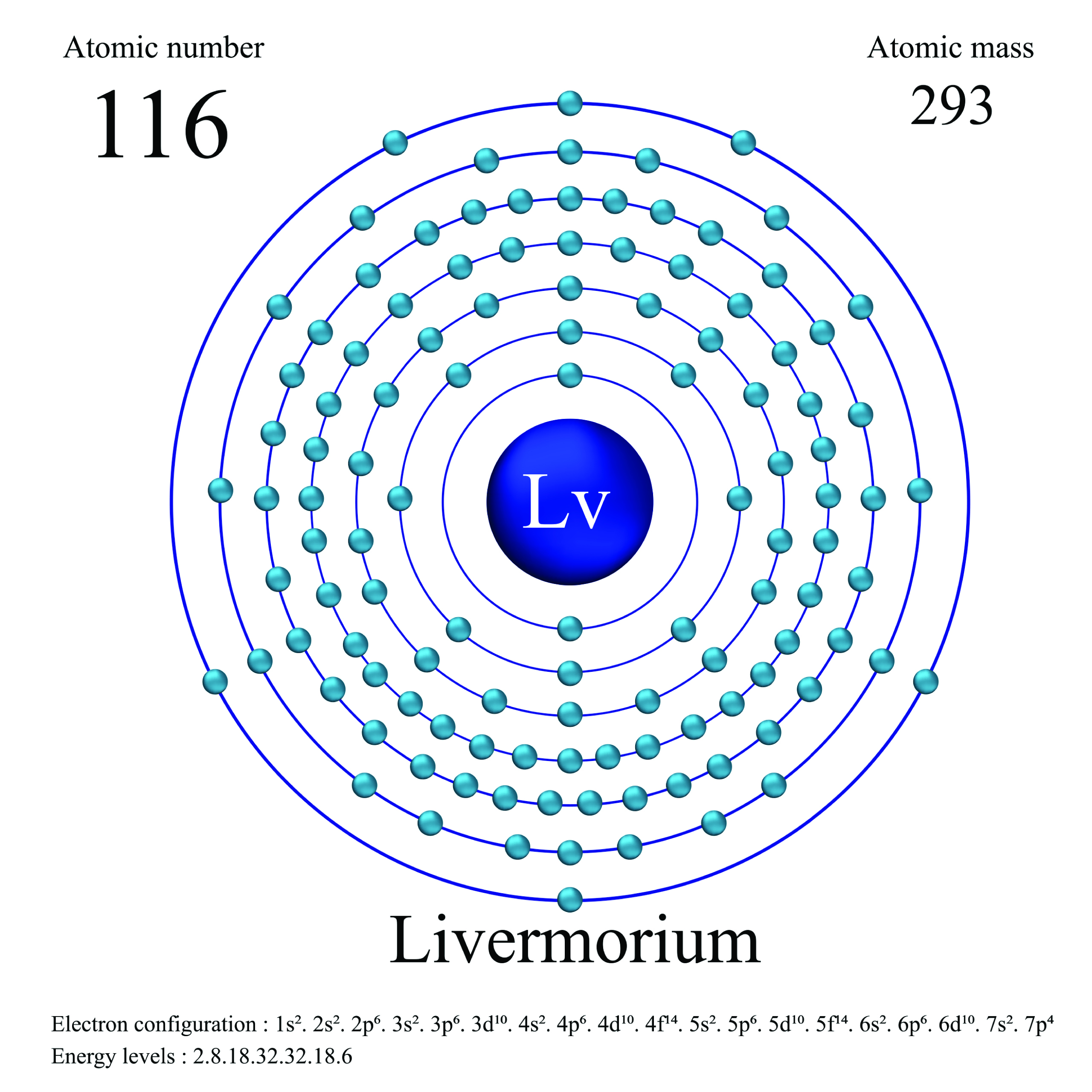
| Name, Symbol | Livermorium, Lv |
| Atomic number | 116 |
| standard atomic mass | [293] |
As with other synthetic elements, livermorium is not found naturally on Earth and can only be produced in a laboratory through nuclear reactions. Its most stable isotope, livermorium-293, has a relatively short half-life of about 60 milliseconds. Due to its limited availability and short half-life, not much is known about the physical and chemical properties of livermorium. Its study is primarily focused on expanding our understanding of superheavy elements and their placement in the periodic table.
4. Moscovium

| Name, Symbol | Moscovium, Mc |
| Atomic number | 115 |
| standard atomic mass | [290] |
Moscovium is a synthetic chemical element with the symbol Mc and atomic number 115. It was first synthesized in 2003 by a team of Russian and American scientists at the Joint Institute for Nuclear Research (JINR) in Dubna, Russia. Like other superheavy elements, moscovium is not found naturally on Earth and can only be produced in a laboratory through nuclear reactions. It is likely to be a highly reactive metal, readily forming compounds with other elements.
5. Flerovium

| Name, Symbol | Flerovium, Fl |
| Atomic number | 114 |
| standard atomic mass | [289] |
Flerovium was first synthesized in 1998 by a joint team of Russian and American scientists at the Joint Institute for Nuclear Research (JINR) in Dubna, Russia, and the Lawrence Livermore National Laboratory in California, USA. Due to its synthetic nature and limited availability, our understanding of flerovium’s properties and characteristics is still evolving. It is likely to have a metallic appearance and exhibit reactivity with certain elements to form compounds.
6. Nihonium
| Name, Symbol | Nihonium, Nh |
| Atomic number | 113 |
| standard atomic mass | [286] |
Nihonium was first synthesized in 2004 by a team of Japanese scientists at the RIKEN Institute in Wako, Japan. As a synthetic element, nihonium is not found naturally on Earth and can only be produced in a laboratory through nuclear reactions. Due to its synthetic nature and limited availability, our knowledge of nihonium’s properties is still evolving. Nihonium belongs to Group 13 on the periodic table, which includes boron, aluminum, gallium, indium, and thallium.
7. Copernicium
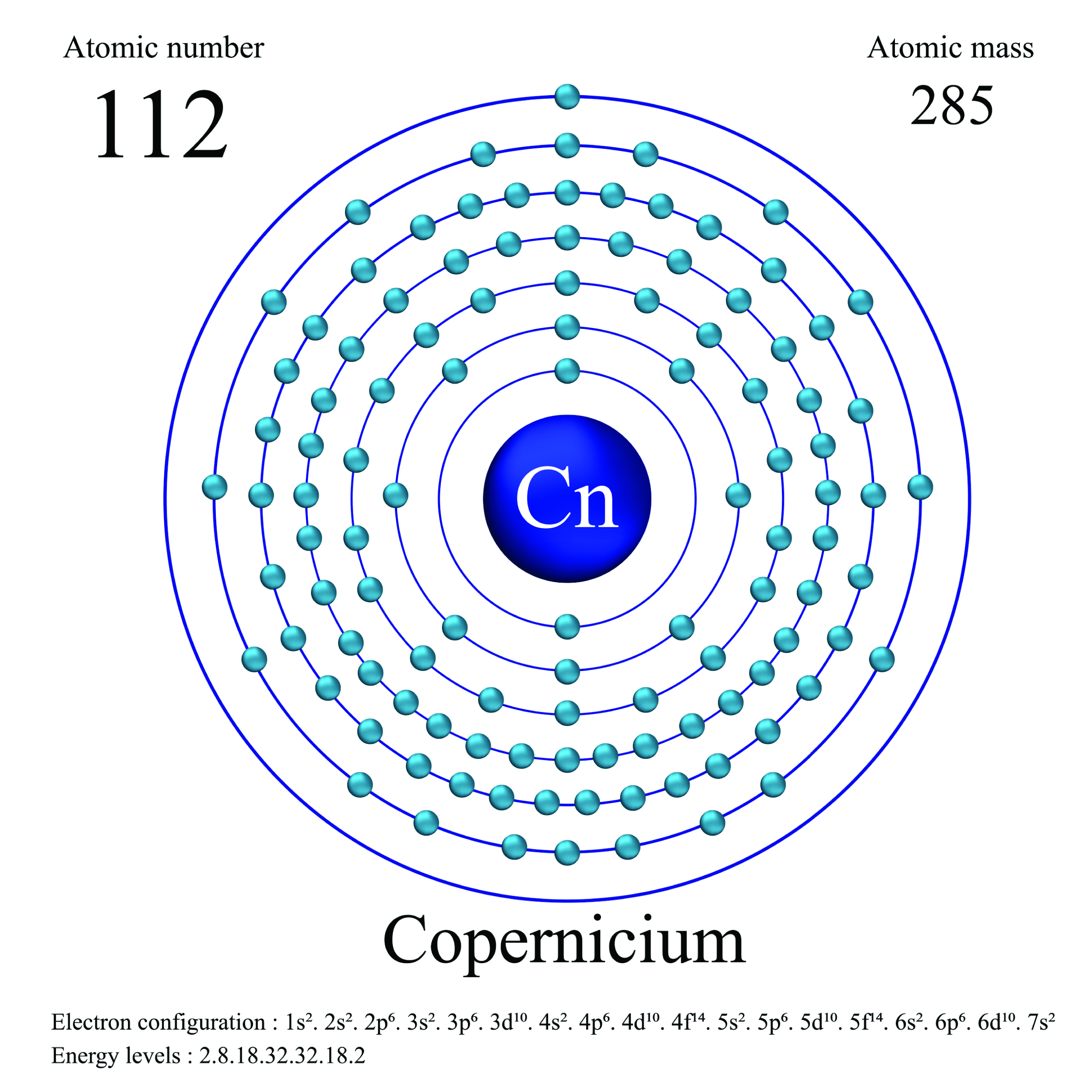
| Name, Symbol | Copernicium, Cn |
| Atomic number | 112 |
| standard atomic mass | [285] |
Copernicium is an extremely heavy and highly radioactive element that was first synthesized in a laboratory in 1996 by a team of German and Russian scientists led by Sigurd Hofmann and Victor Ninov. It is classified as a transactinide element, which means it is part of a group of elements that are very difficult to synthesize and have extremely short half-lives. Its melting and boiling points are unknown, but they are likely to be high due to the heavy nature of the element.
8. Roentgenium

| Name, Symbol | Roentgenium, Rg |
| Atomic number | 111 |
| standard atomic mass | [282] |
Roentgenium is an extremely heavy and highly radioactive element that was first synthesized in a laboratory in 1994 by a team of German and Russian scientists led by Sigurd Hofmann and Victor Ninov. Roentgenium has an atomic number of 111, which means it has 111 protons in its nucleus. As an extremely rare and highly radioactive element, Roentgenium currently has no practical applications. Its primary significance lies in advancing scientific knowledge about the properties of heavy and superheavy elements.
9. Darmstadtium (Ds)
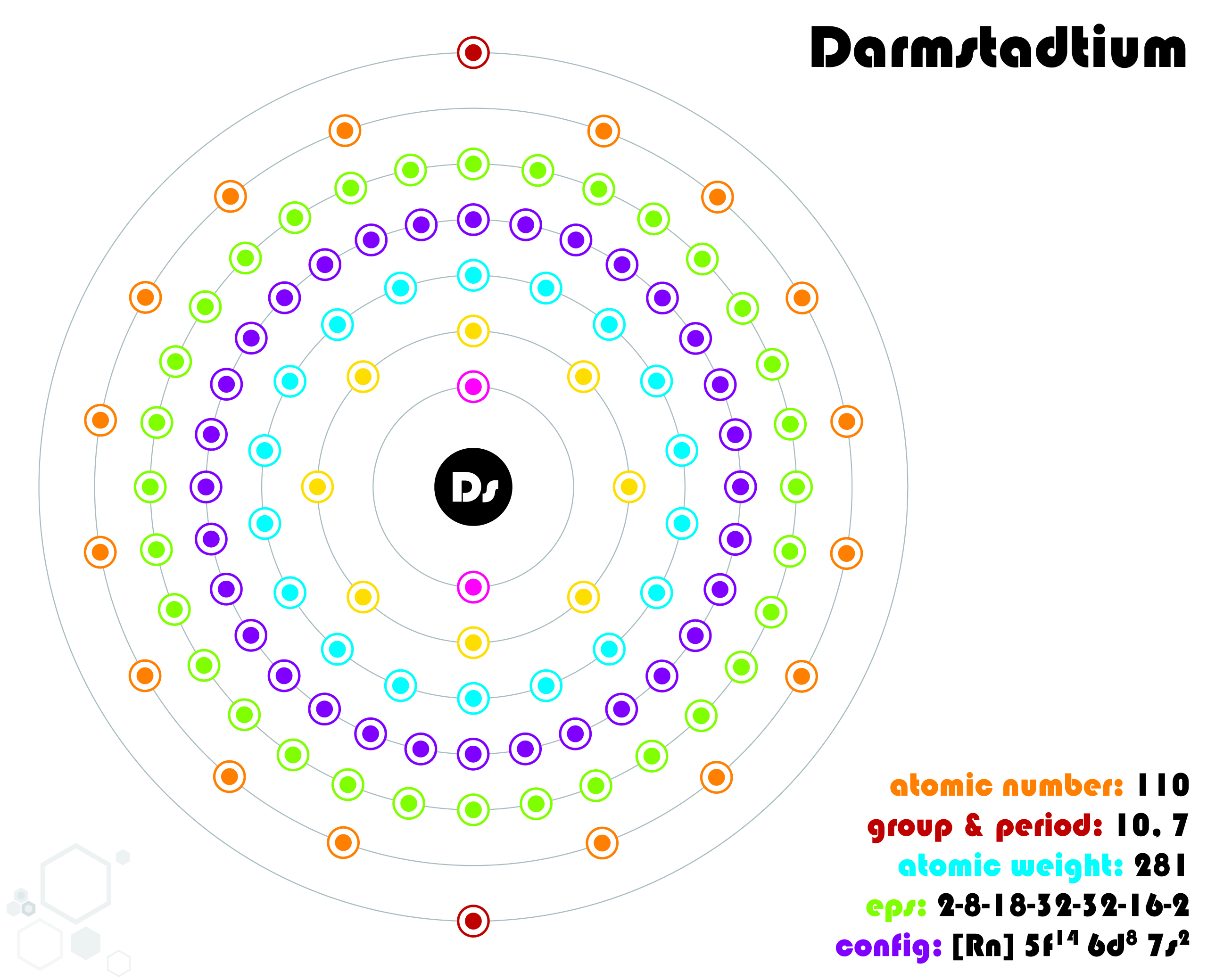
| Name, Symbol | Darmstadtium, Ds |
| Atomic number | 110 |
| standard atomic mass | [281] |
Darmstadtium is an extremely heavy and highly radioactive element that was first synthesized in a laboratory in 1994 by a team of German scientists led by Sigurd Hofmann at the GSI Helmholtz Centre for Heavy Ion Research in Darmstadt, Germany. It is classified as a transactinide element, which means it is part of a group of elements that are very difficult to synthesize and have extremely short half-lives. Its melting and boiling points are unknown, but they are likely to be high due to the heavy nature of the element.
10. Meitnerium (Mt)
| Name, Symbol | Meitnerium, Mt |
| Atomic number | 109 |
| standard atomic mass | [278] |
Meitnerium is an extremely heavy and highly radioactive element that was first synthesized in a laboratory in 1982 by a team of German scientists led by Peter Armbruster and Gottfried Münzenberg at the Institute for Heavy Ion Research (GSI) in Darmstadt, Germany. It is classified as a transactinide element, which means it is part of a group of elements that are very difficult to synthesize and have extremely short half-lives. Its primary significance lies in advancing scientific knowledge about the properties of heavy and superheavy elements.
11. Seaborgium
| Name, Symbol | Seaborgium, Sg |
| Atomic number | 106 |
| standard atomic mass | [269] |
Seaborgium is an extremely heavy and highly radioactive element that was first synthesized in a laboratory in 1974 by a team of American scientists led by Albert Ghiorso at the Lawrence Berkeley National Laboratory in California.Seaborgium has an atomic number of 106, which means it has 106 protons in its nucleus. Its atomic weight is believed to be around 266 atomic mass units. Its melting and boiling points are unknown, but they are likely to be high due to the heavy nature of the element. As an extremely rare and highly radioactive element, Seaborgium currently has no practical applications.
12. Hassium (Hs)
| Name, Symbol | Hassium, Hs |
| Atomic number | 108 |
| standard atomic mass | [269] |
Hassium is one of the transactinide elements, which are extremely heavy elements located beyond uranium on the periodic table. As a synthetic element, hassium has not been sufficiently produced to measure its physical properties accurately. Hassium is not found naturally on Earth and is exclusively produced in laboratories through nuclear reactions. Its primary significance lies in expanding our understanding of nuclear physics and the properties of superheavy elements.
13. Dubnium (Db)
| Name, Symbol | Dubnium, Db |
| Atomic number | 105 |
| standard atomic mass | [268] |
Dubnium is named after the Joint Institute for Nuclear Research in Dubna, Russia, where it was first synthesized. Due to its high atomic number, dubnium is predicted to be highly reactive and form various chemical compounds. It is typically synthesized by bombarding a target material, such as californium or berkelium, with a beam of high-energy particles, such as ions.
14. Rutherfordium (Rf)
| Name, Symbol | Rutherfordium, Rf |
| Atomic number | 104 |
| standard atomic mass | [267] |
Rutherfordium is named after Ernest Rutherford, a prominent physicist and Nobel laureate. It is expected to be a solid at room temperature, have a metallic appearance, and exhibit properties similar to other transition metals. The limited availability and short half-life of rutherfordium isotopes have hindered detailed experimental studies of its chemical behavior. The resulting isotopes of rutherfordium are highly unstable and quickly decay into other elements.
15. Lawrencium (Lr)

| Name, Symbol | Lawrencium, Lr |
| Atomic number | 103 |
| standard atomic mass | [266] |
Lawrencium belongs to the actinide series of elements and is classified as a transactinide element. Lawrencium is presumed to be a silvery-white metal, but its precise appearance and other physical properties are still under investigation. Due to its high atomic number, lawrencium is expected to be highly reactive and form various chemical compounds. Lawrencium’s practical applications are limited due to its short half-life and limited availability.
16. Nobelium (No)
| Name, Symbol | Nobelium, No |
| Atomic number | 102 |
| standard atomic mass | [259] |
Nobelium is named after Alfred Nobel, the Swedish chemist, and inventor of dynamite. As a synthetic element, the physical properties of nobelium have not been precisely determined. It is typically synthesized by bombarding a target material, such as curium or plutonium, with a beam of high-energy particles, such as ions. Its primary significance lies in scientific research, particularly in the study of nuclear physics, the behavior of superheavy elements, and the exploration of the periodic table.
17. Mendelevium (Md)
| Name, Symbol | Mendelevium, Md |
| Atomic number | 101 |
| standard atomic mass | [258] |
Mendelevium belongs to the actinide series of elements and is classified as a transactinide element. Mendelevium is a highly radioactive element, and all of its isotopes are unstable. As a synthetic element, the physical properties of mendelevium have not been extensively studied. Mendelevium is not found naturally on Earth and is exclusively produced in laboratories through nuclear reactions. Further research is required to gain a more comprehensive understanding of mendelevium’s properties.
18. Fermium (Fm)
| Name, Symbol | Fermium, Fm |
| Atomic number | 100 |
| standard atomic mass | [257] |
Fermium belongs to the actinide series of elements and is classified as a transactinide element. Fermium is not found naturally on Earth and is exclusively produced in laboratories through nuclear reactions. It is typically synthesized by bombarding a target material, such as plutonium or curium, with a beam of high-energy particles, such as ions. Its primary significance lies in scientific research, particularly in the study of nuclear physics, the behavior of superheavy elements, and the exploration of the periodic table.
19. Einsteinium (Es)
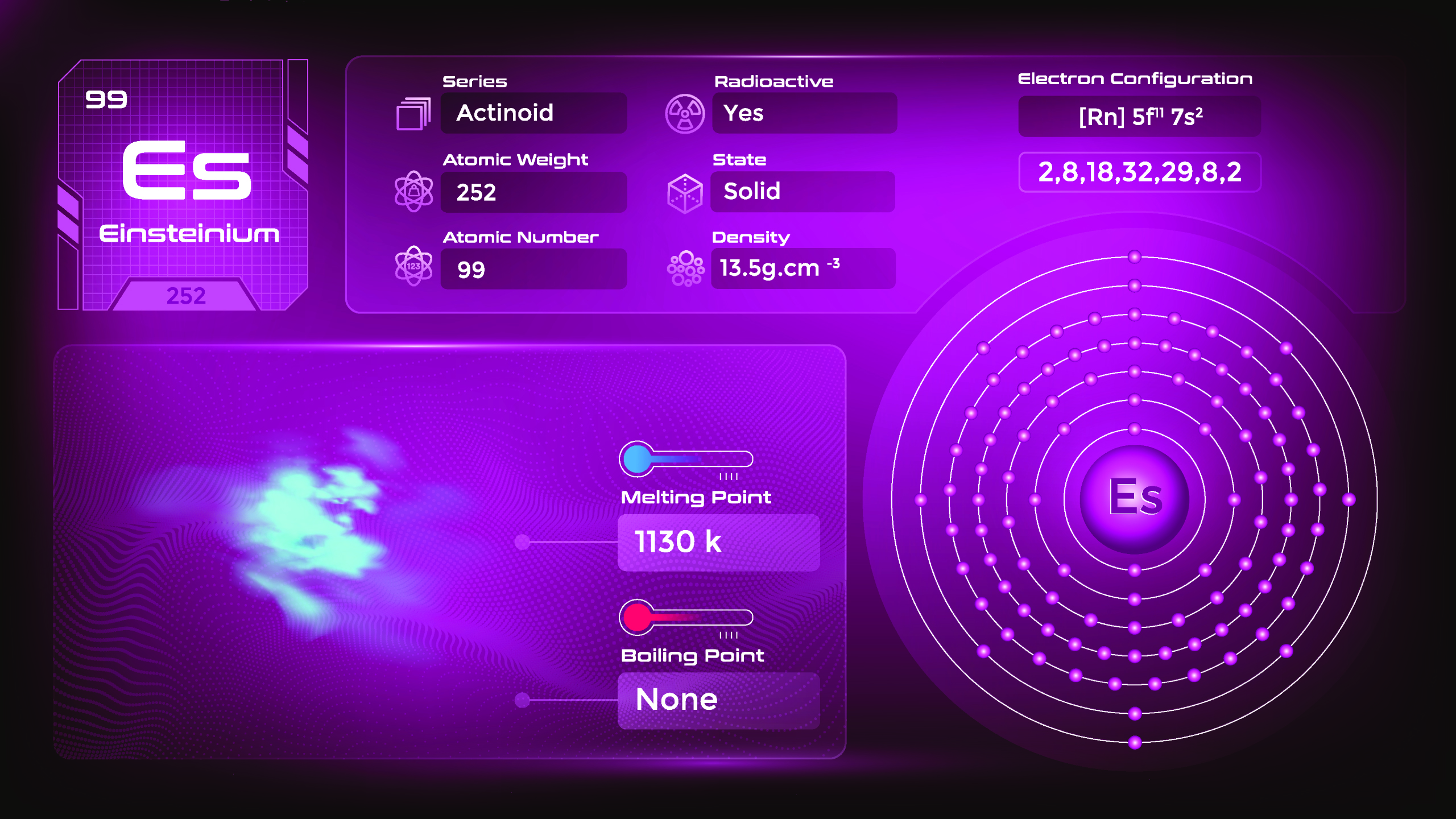
| Name, Symbol | Einsteinium, Es |
| Atomic number | 99 |
| standard atomic mass | [252] |
Einsteinium is named after Albert Einstein, the renowned physicist who formulated the theory of relativity. Its most stable isotope, einsteinium-252, has a half-life of approximately 471.7 days. As a synthetic element, the physical properties of einsteinium have not been extensively studied. Due to its short half-life and limited availability, einsteinium has limited practical applications outside of scientific research.
20. Californium (Cf)
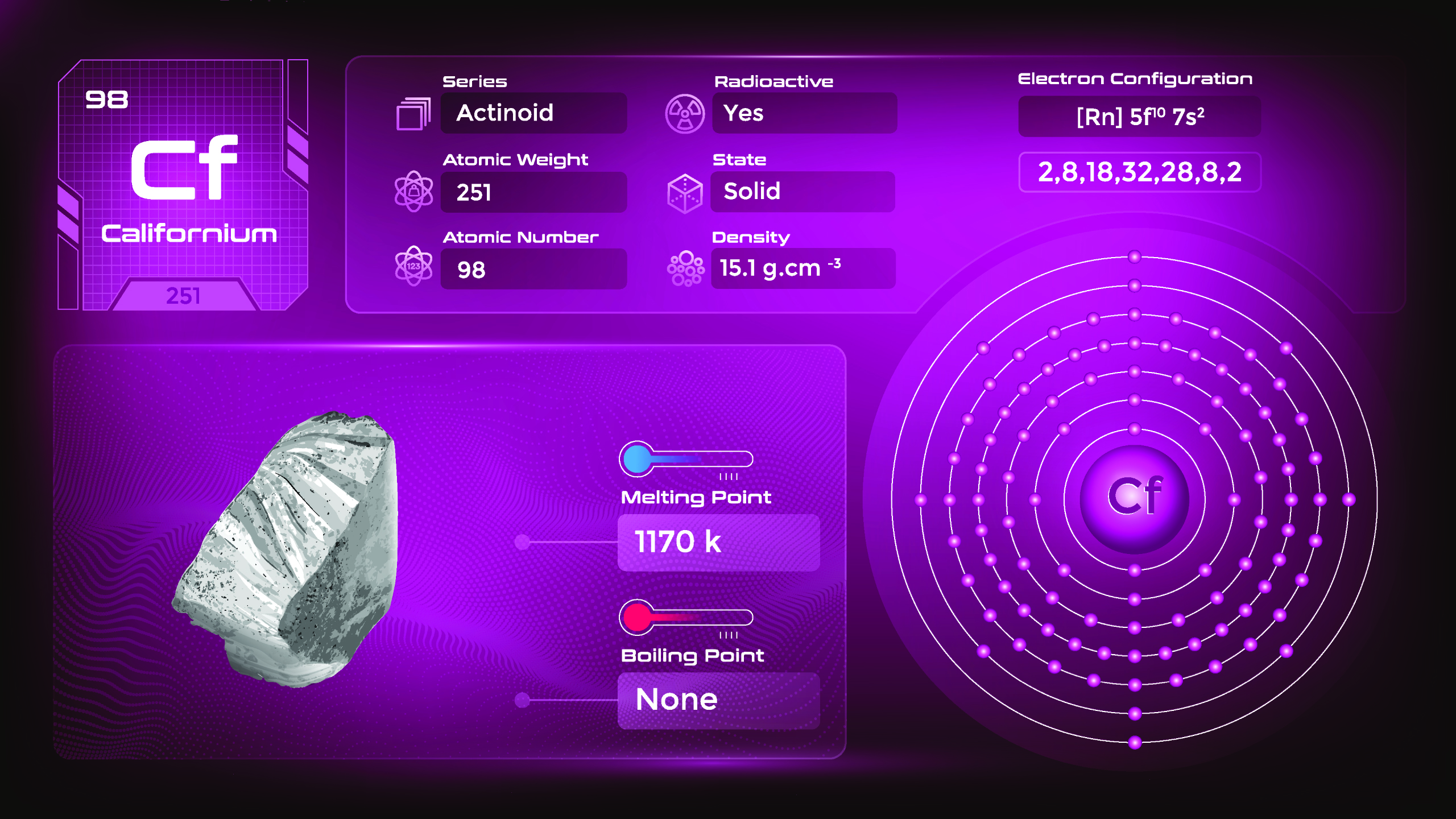
| Name, Symbol | Californium, Cf |
| Atomic number | 98 |
| standard atomic mass | [251] |
Californium is named after the state of California, where it was first synthesized. As a synthetic element, the physical properties of californium have not been extensively studied. Californium’s chemical properties are predicted to be similar to other actinide elements in the periodic table. It is not found naturally on Earth and is exclusively produced in laboratories through nuclear reactions.
21. Berkelium (Bk)
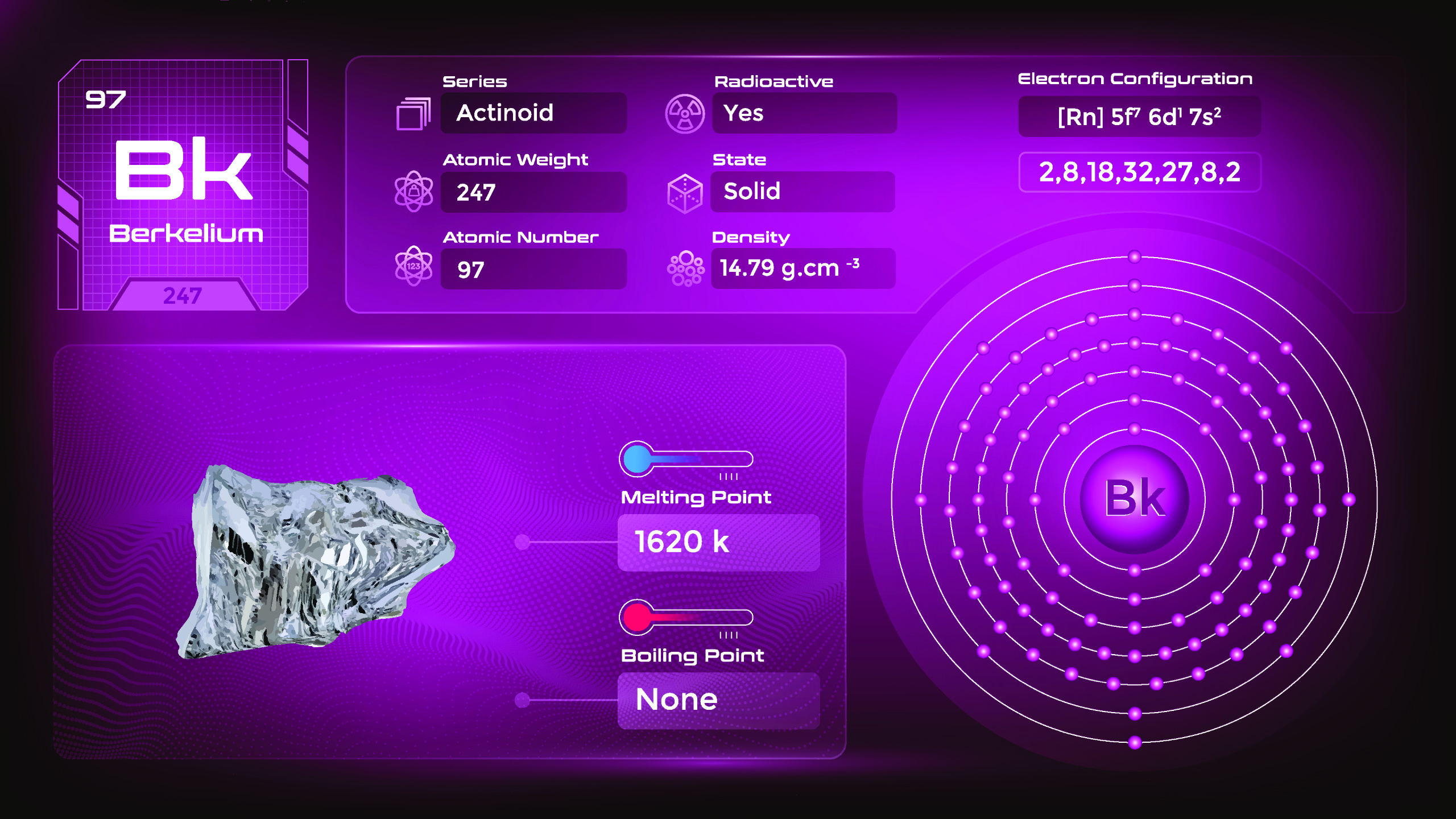
| Name, Symbol | Berkelium, Bk |
| Atomic number | 97 |
| standard atomic mass | [247] |
Berkelium belongs to the actinide series of elements and is named after the city of Berkeley, California, where it was first synthesized. Berkelium is a radioactive metal and is silver-gray in color. It is solid at room temperature and has a relatively high melting point. Berkelium is produced in nuclear reactors by bombarding heavy isotopes of other elements with neutrons. It is primarily obtained through the nuclear transmutation of the element americium.
22. Curium (Cm)
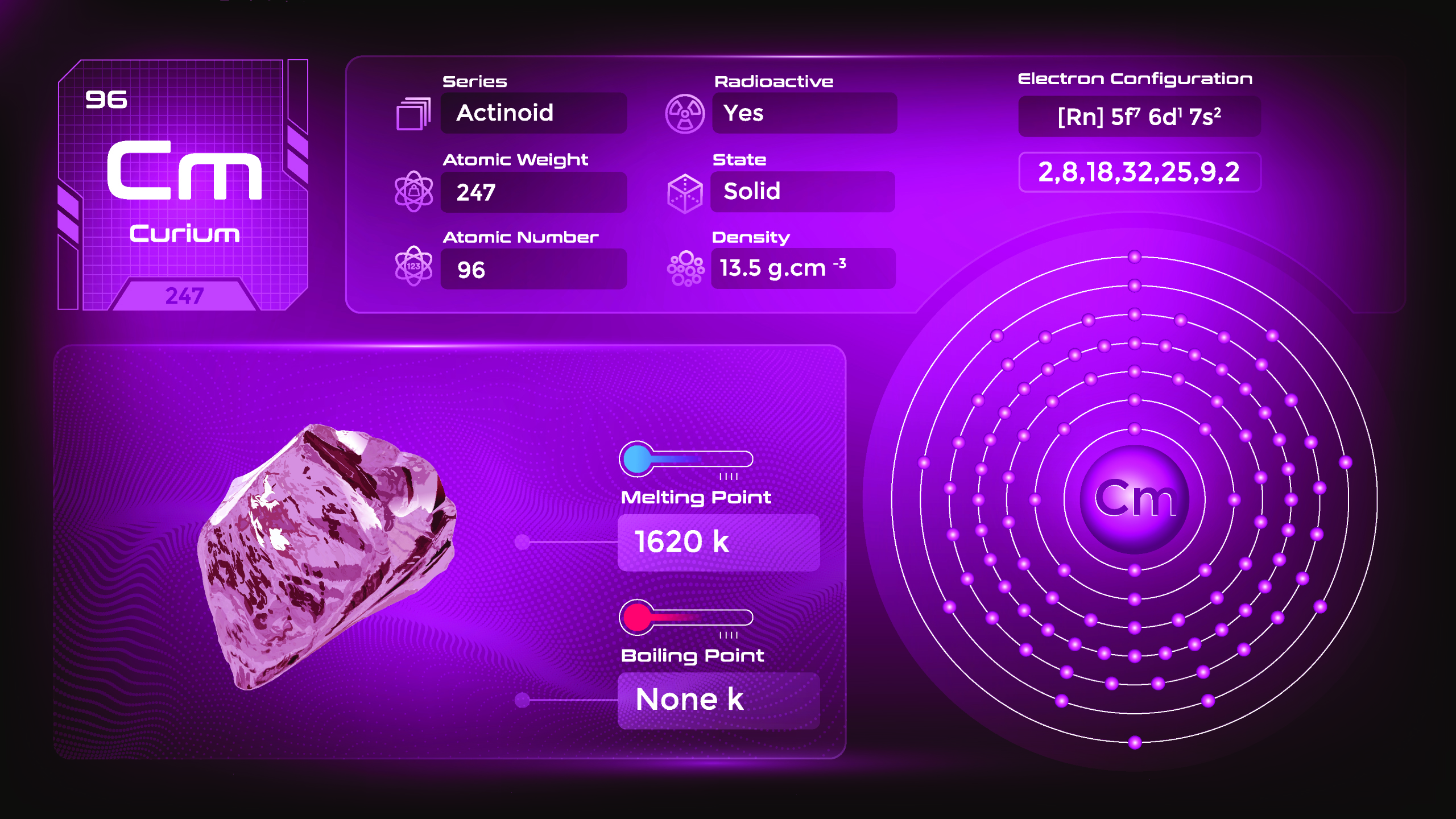
| Name, Symbol | Curium, Cm |
| Atomic number | 96 |
| standard atomic mass | [247] |
Curium is a member of the actinide series of elements and is produced through nuclear reactions in a laboratory. Curium is a radioactive metal and is silvery-white in color. It is solid at room temperature and has a relatively high melting and boiling point. Curium is a highly reactive element, readily forming compounds with various elements. They emit ionizing radiation and should be handled with extreme caution in specialized laboratories following strict safety protocols.
23. Plutonium (Pu)
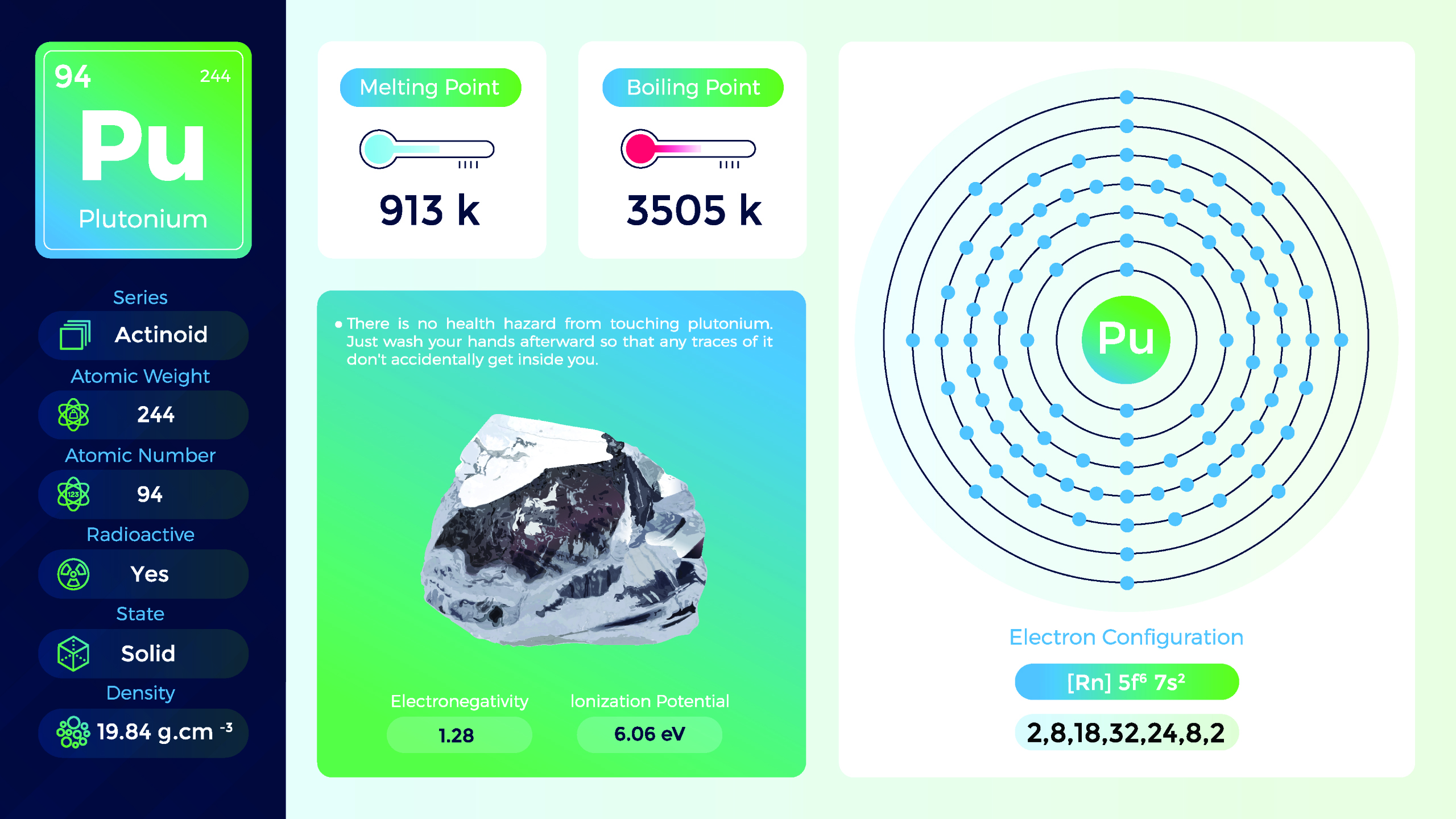
| Name, Symbol | Plutonium, Pu |
| Atomic number | 94 |
| standard atomic mass | [244] |
Plutonium is a member of the actinide series of elements and is named after the planet Pluto. Plutonium is a dense, silvery metal at room temperature. It is malleable and can be easily shaped or formed. The most common isotopes of plutonium are plutonium-239, plutonium-240, and plutonium-241. Handling plutonium requires strict safety protocols and specialized facilities to prevent exposure.
24. Americium (Am)
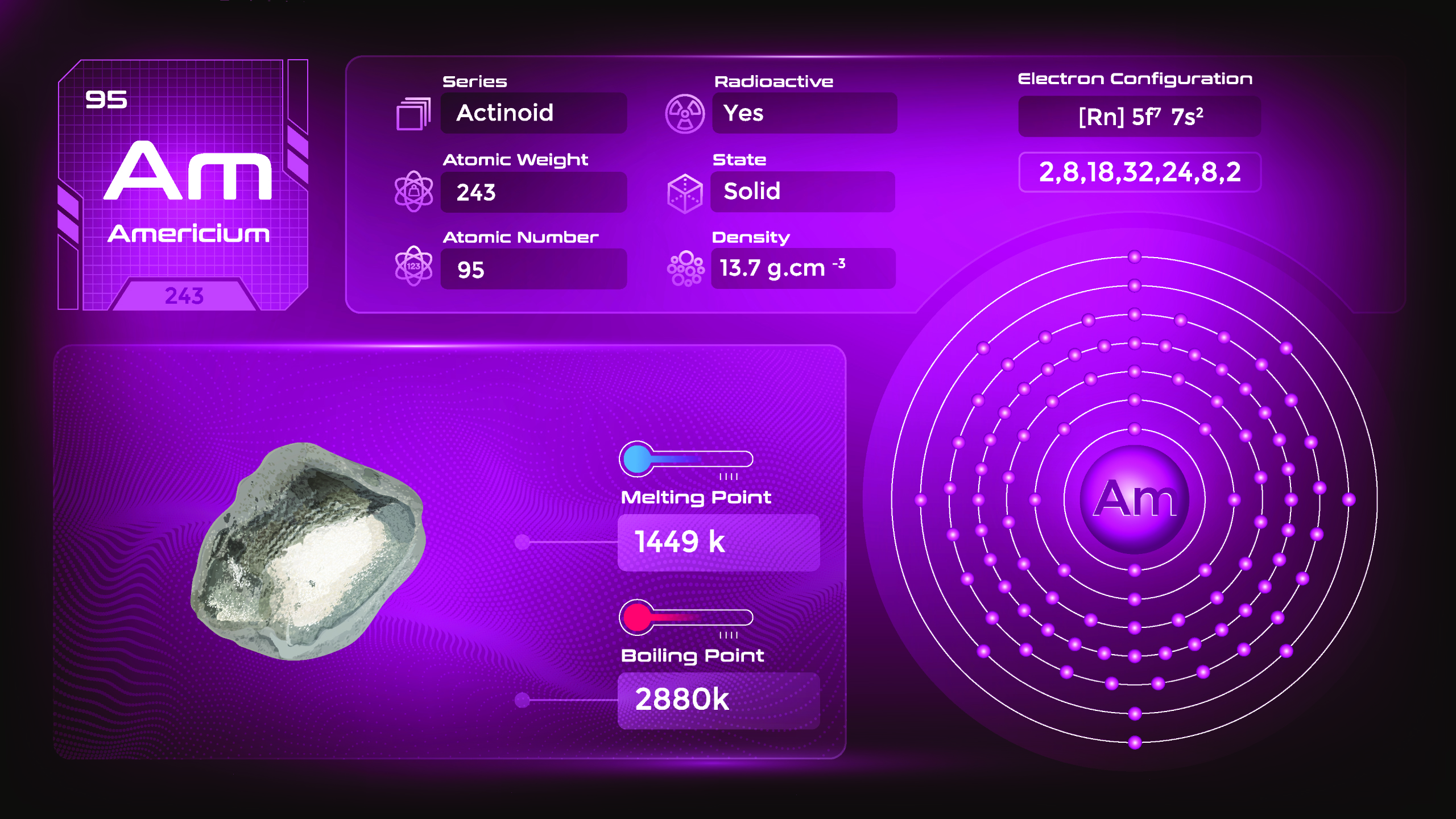
| Name, Symbol | Americium, Am |
| Atomic number | 95 |
| standard atomic mass | [243] |
Americium is a silvery-white metal at room temperature. It is relatively dense, malleable, and has a high melting point. Americium is paramagnetic, meaning it is weakly attracted to magnetic fields. Americium-241 is used in smoke detectors, where it emits alpha particles that ionize the air in the presence of smoke, triggering the alarm. It can also be obtained as a byproduct of uranium or plutonium nuclear fuel.
25. Uranium (U)
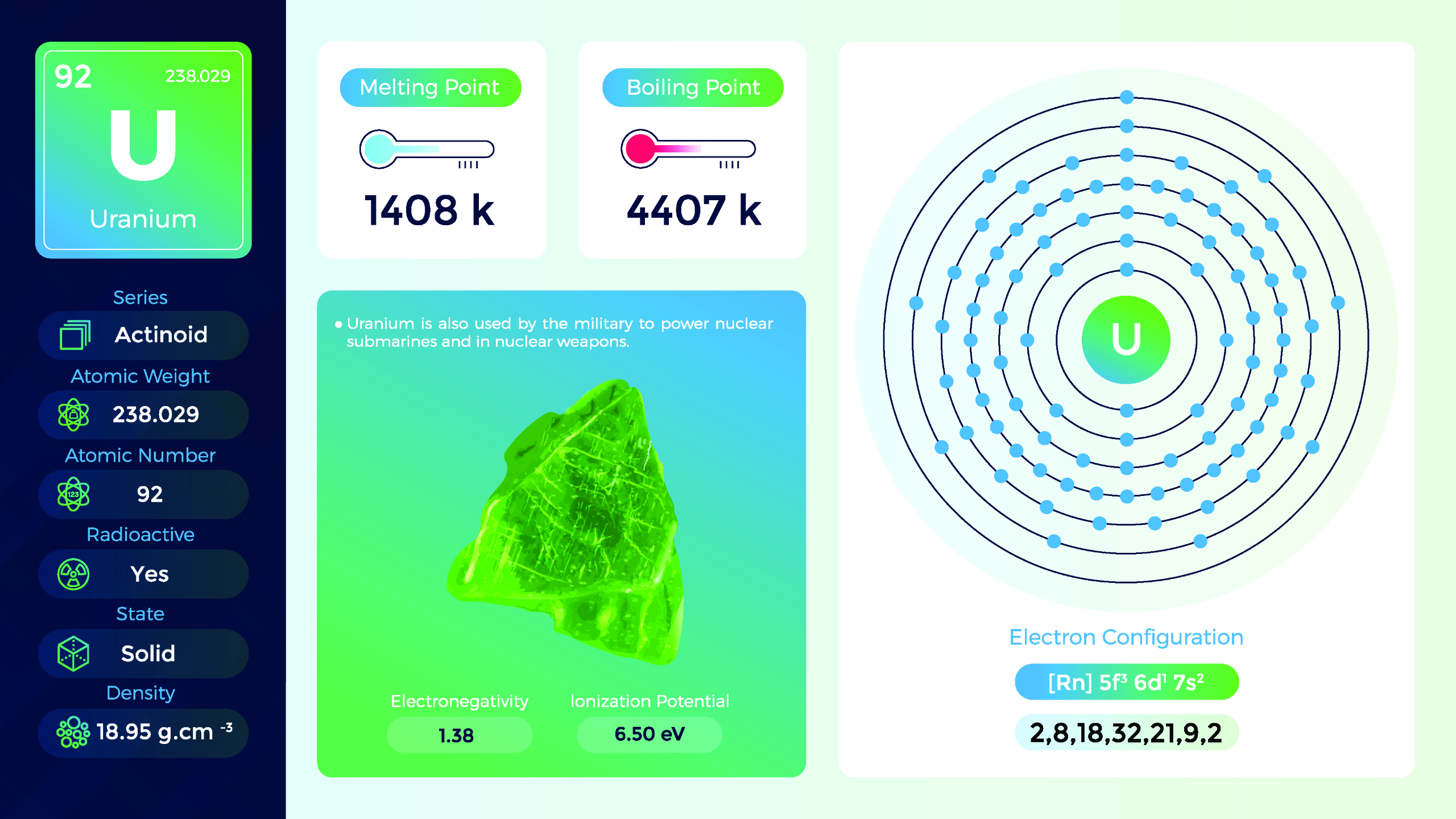
| Name, Symbol | Uranium, U |
| Atomic number | 92 |
| standard atomic mass | 238.03 |
Uranium is a member of the actinide series of elements and is widely known for its use as fuel in nuclear reactors and for its radioactive properties. Uranium is a dense, silvery-gray metal. It is malleable, ductile, and relatively soft compared to most metals. Proper handling, storage, and disposal procedures are essential to prevent exposure and minimize the associated risks. The extraction, production, and disposal of uranium and its byproducts require adherence to stringent safety and environmental standards.
26. Neptunium (Np)
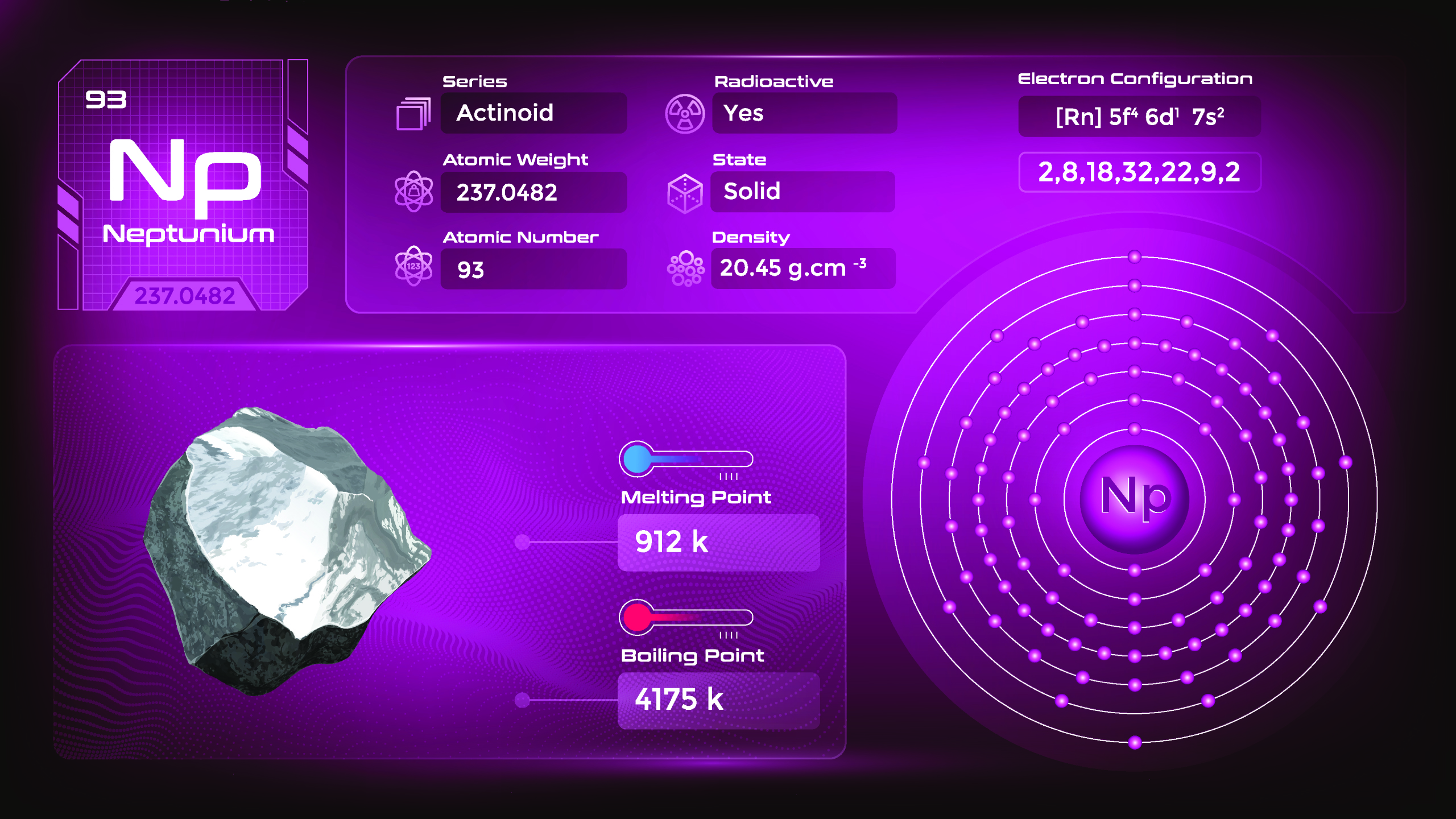
| Name, Symbol | Neptunium, Np |
| Atomic number | 93 |
| standard atomic mass | [237] |
Neptunium is a silvery metal at room temperature. It is relatively dense, malleable, and ductile. Neptunium has a high melting point and is a poor conductor of electricity. It emits alpha particles, beta particles, and gamma rays during radioactive decay. They emit ionizing radiation and should be handled with great care in specialized laboratories and facilities. Its properties and behavior help researchers understand the nature of heavy elements and their role in nuclear reactions.
27. Thorium (Th)
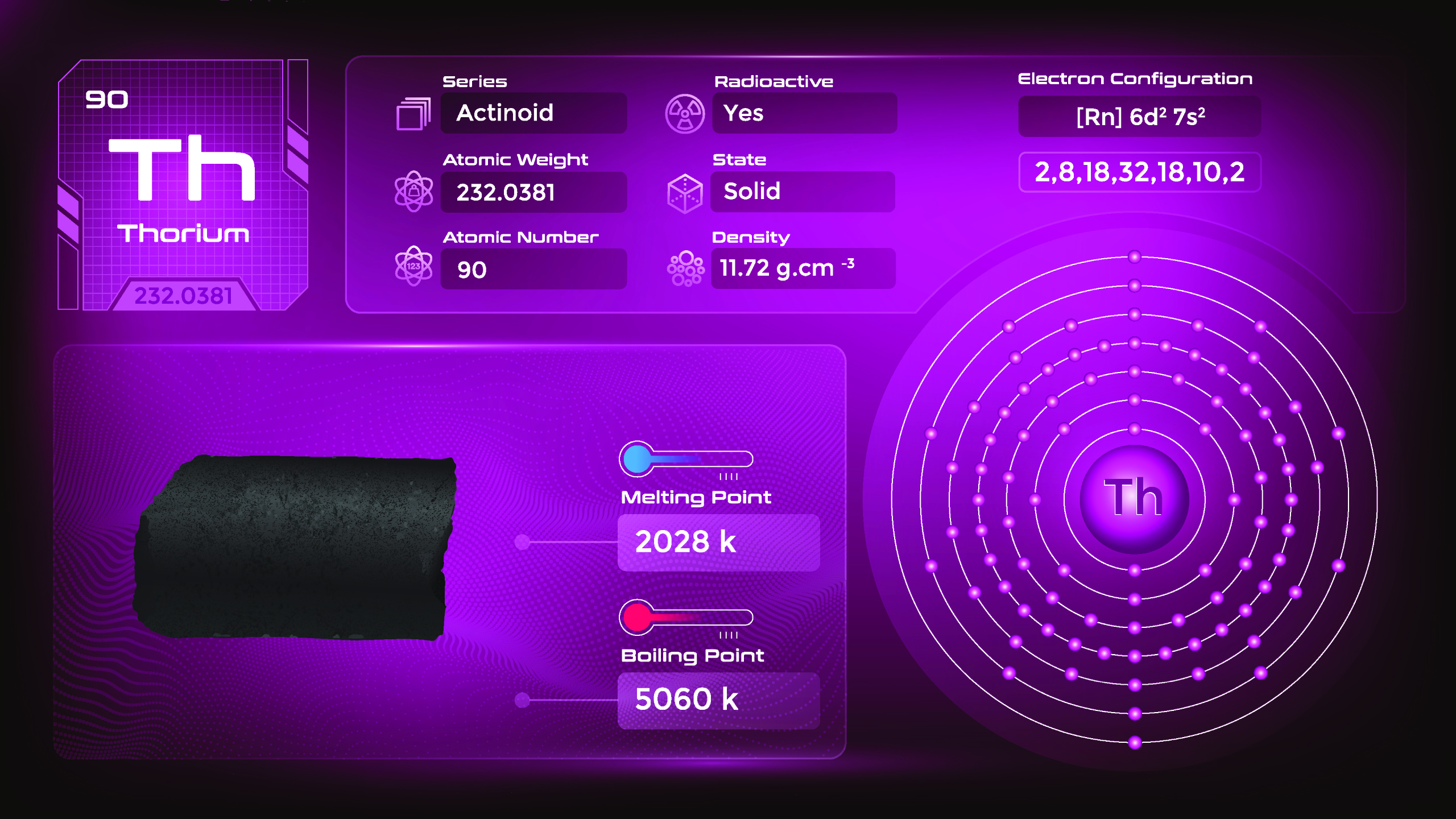
| Name, Symbol | Thorium, Th |
| Atomic number | 90 |
| standard atomic mass | 232.04 |
Thorium is a silvery-white, radioactive metal. It is malleable, ductile, and relatively soft compared to other metals. Thorium has a high melting point and is paramagnetic, meaning it is weakly attracted to magnetic fields. It undergoes spontaneous radioactive decay, emitting alpha particles, beta particles, and gamma rays. Thorium compounds include oxides, halides, and various complex ions. They emit ionizing radiation and should be handled with care in specialized laboratories and facilities.
28. Protactinium (Pa)
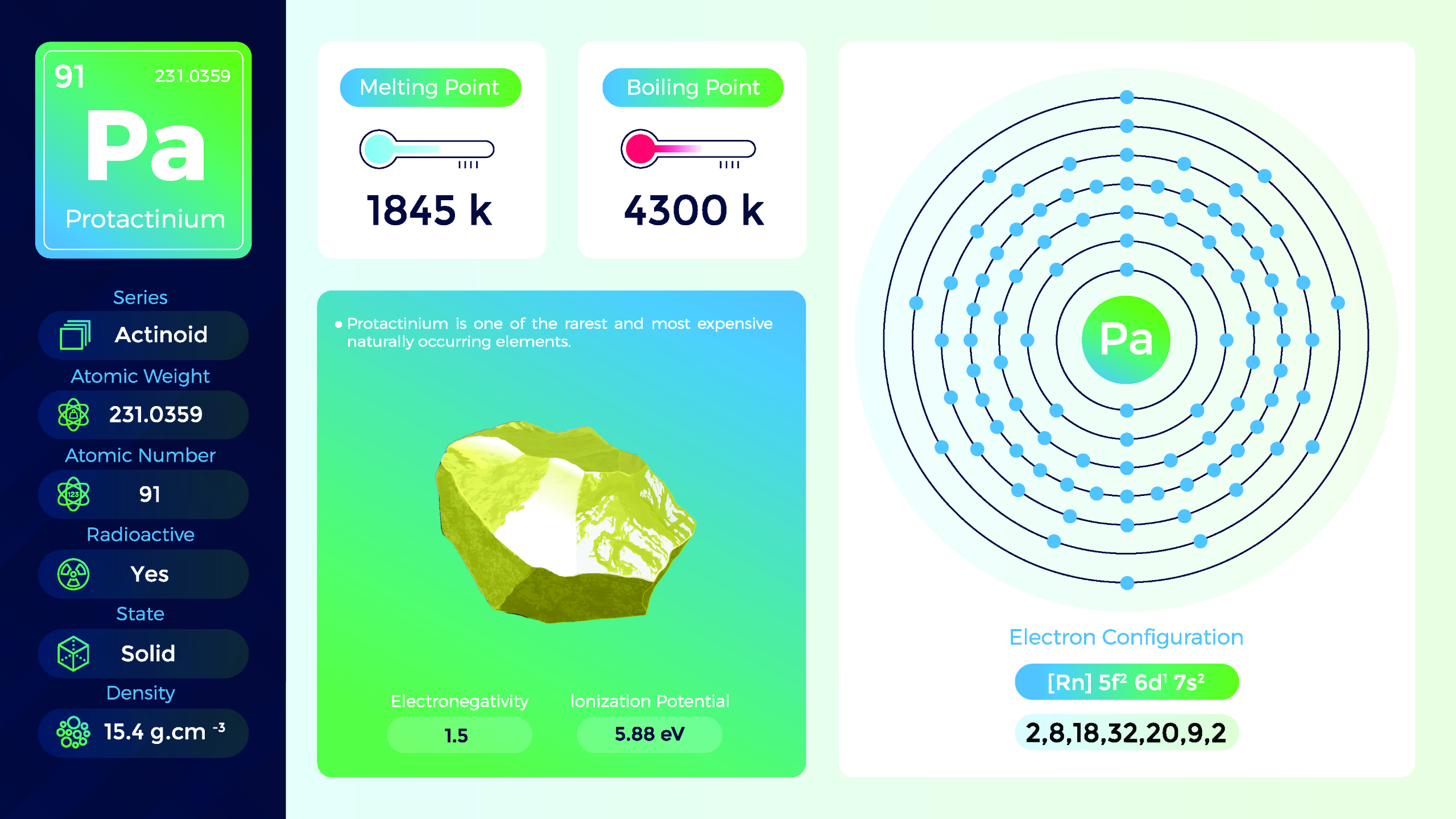
| Name, Symbol | Protactinium, Pa |
| Atomic number | 91 |
| standard atomic mass | 231,04 |
Protactinium is a dense, silvery-gray metal. It is malleable and ductile, and its physical properties are similar to those of other actinide elements. Protactinium is primarily produced in nuclear reactors by bombarding uranium or thorium with neutrons. They emit ionizing radiation and should be handled with great care in specialized laboratories and facilities. Strict safety protocols are necessary to prevent exposure and protect against radiation hazards.
29. Actinium (Ac)
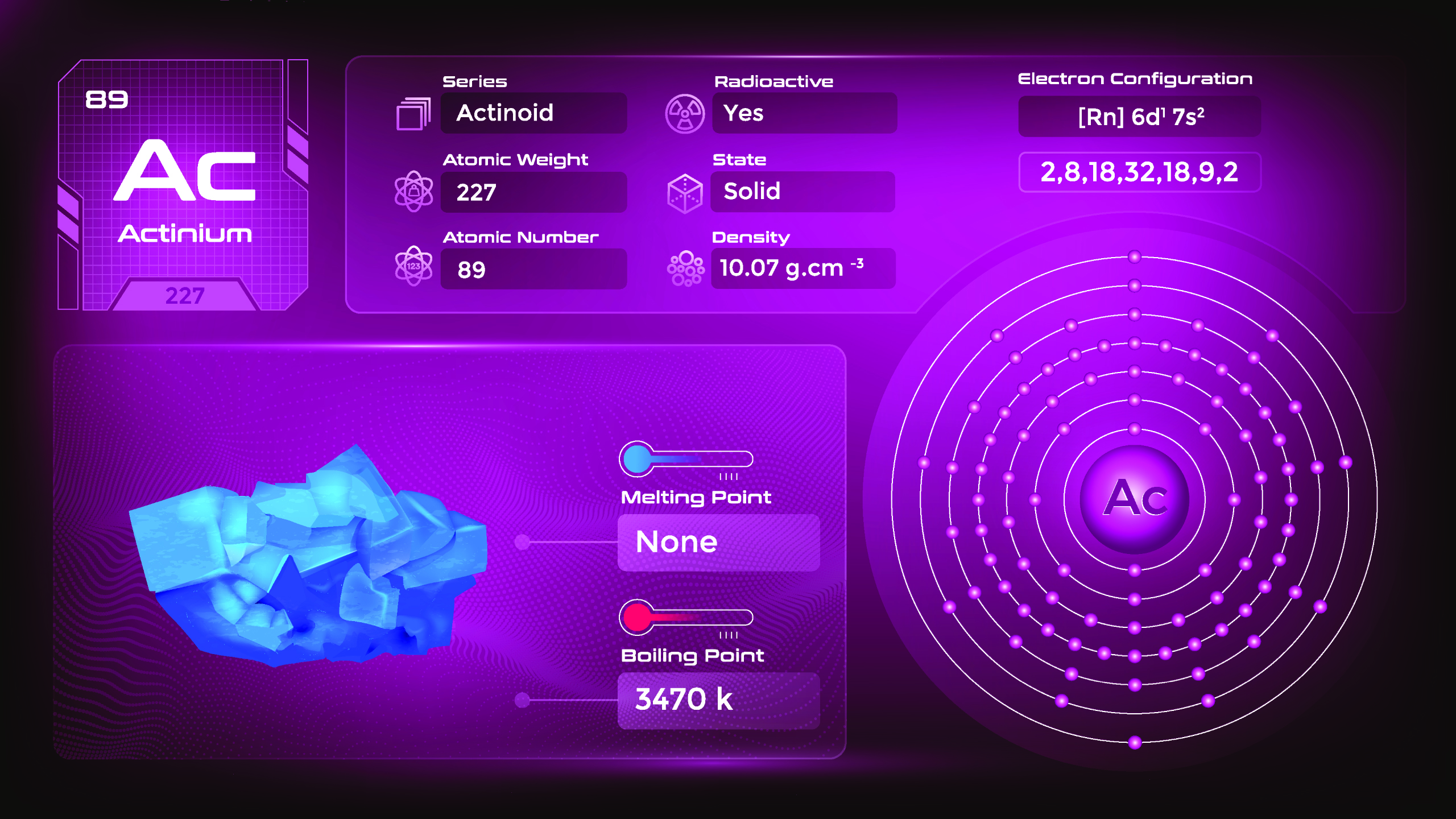
| Name, Symbol | Actinium, Ac |
| Atomic number | 89 |
| standard atomic mass | [227] |
Actinium is a silvery-white metal. It is dense, malleable, and relatively soft. Actinium has a high melting point and is a poor conductor of electricity. It is primarily found as a decay product of uranium and thorium in trace amounts in uranium ores. They emit ionizing radiation and should be handled with great care in specialized laboratories and facilities. Due to its radioactivity and limited availability, its practical applications are limited.
30. Radium (Ra)

| Name, Symbol | Radium, Ra |
| Atomic number | 88 |
| standard atomic mass | [226] |
Radium belongs to the alkaline earth metal group and is known for its intense radioactivity. Radium is a silvery-white, lustrous metal. It reacts vigorously with water, corrodes in air, and forms various compounds, mainly as radium salts. They emit ionizing radiation, primarily alpha particles, which can damage tissues and organs if ingested, inhaled, or come into direct contact with the body.
31. Francium (Fr)
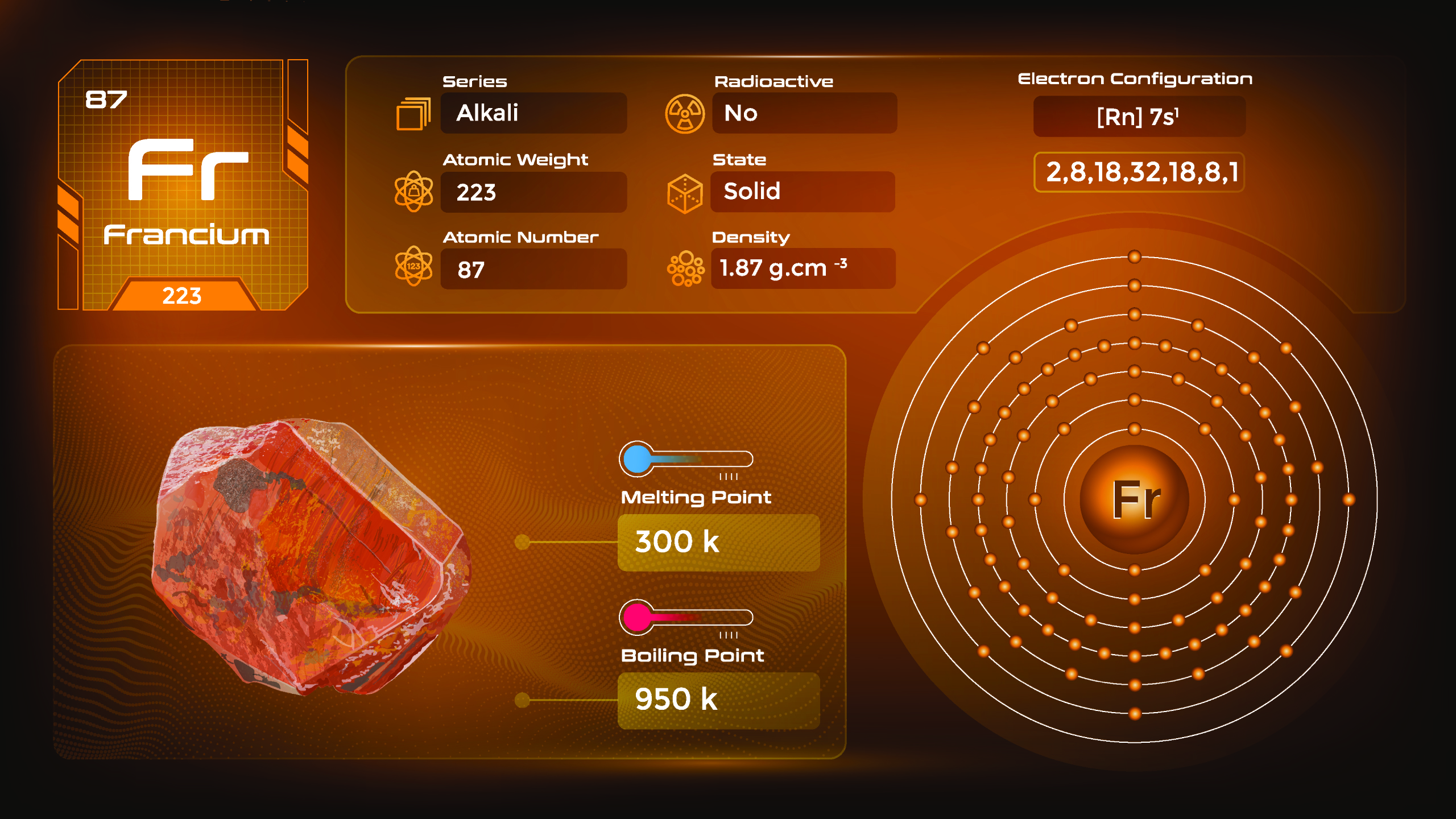
| Name, Symbol | Francium, Fr |
| Atomic number | 87 |
| standard atomic mass | [223] |
Francium is the second-rarest naturally occurring element in the Earth’s crust, and its most stable isotope has a very short half-life. Francium is a highly reactive alkali metal and is expected to have a silvery-white appearance. Due to its extreme rarity and highly radioactive nature, francium has no practical applications. Handling francium requires strict safety protocols and specialized facilities.
32. Astatine (At)
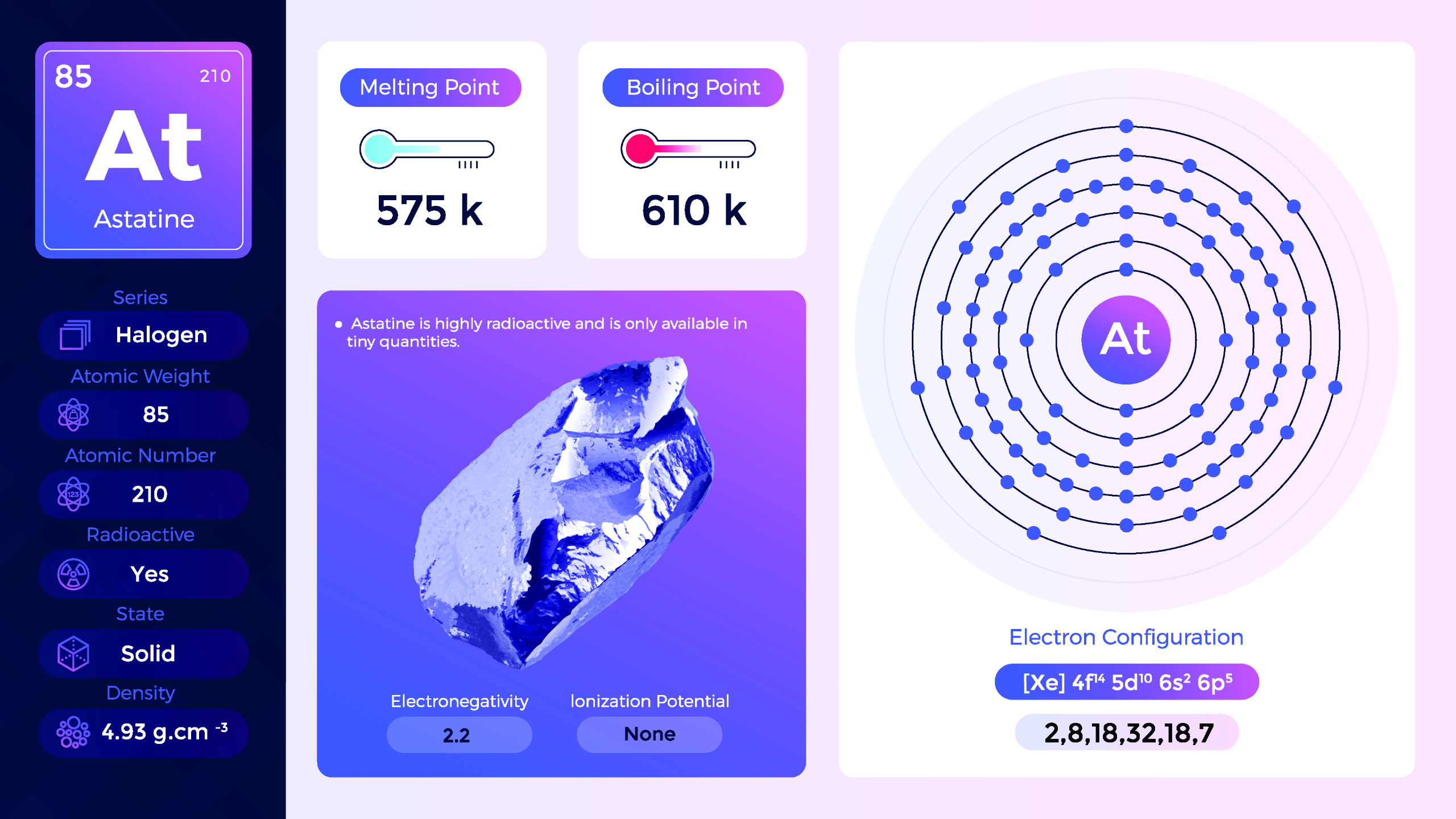
| Name, Symbol | Astatine, At |
| Atomic number | 85 |
| standard atomic mass | [210] |
Astatine is a highly radioactive chemical element with the symbol At and atomic number 85. Astatine is a dark, solid element that is rarely observed in its pure form. It is usually described as either a black solid or a metallic-looking solid with a high luster. However, due to its rarity and radioactivity, astatine’s chemical properties have not been extensively studied. Proper shielding and strict safety protocols are necessary to minimize radiation exposure.
33. Polonium (Po)
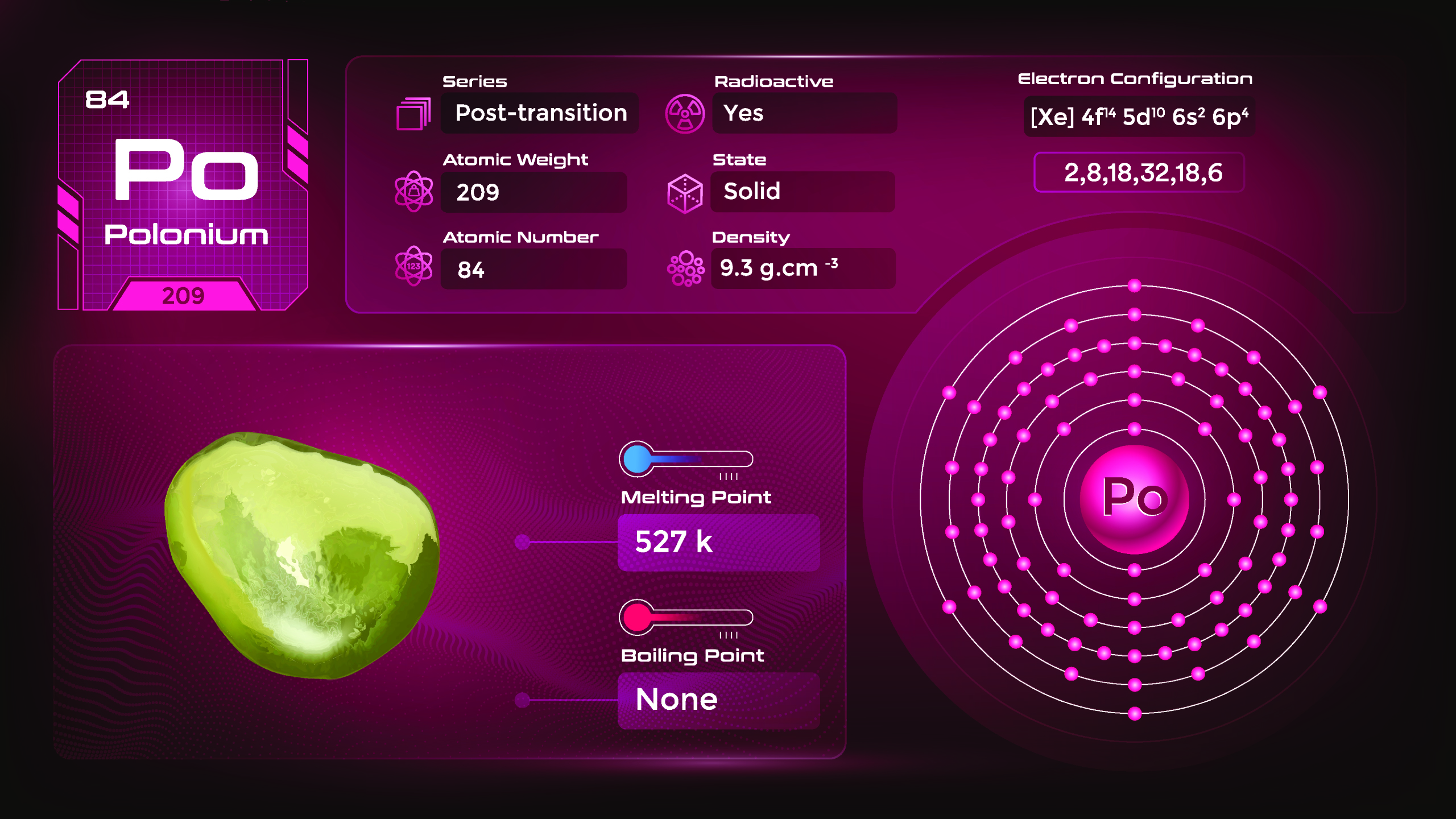
| Name, Symbol | Polonium, Po |
| Atomic number | 84 |
| standard atomic mass | [209] |
Polonium is a silvery-gray metal with a metallic luster. It is a poor conductor of heat and electricity. Polonium has a relatively low melting point and is highly volatile. It can exhibit various oxidation states, including +2 and +4. Polonium compounds include oxides, halides, and various organic compounds. They emit ionizing radiation and pose a significant health hazard. Handling polonium requires strict safety protocols and specialized facilities to prevent exposure.
34. Bismuth (Bi)

| Name, Symbol | Bismuth, Bi |
| Atomic number | 83 |
| standard atomic mass | 208,99 |
Bismuth is a brittle metal with a silvery-white color. Due to its low density, bismuth is often used in applications where a low-mass material is desired. Bismuth alloys are used in low-melting-point solders, as well as in fire sprinkler systems due to their ability to melt and release water when exposed to high temperatures. Its applications range from pharmaceuticals to soldering, and it continues to be an important element in various industries.
35. Lead (Pb)
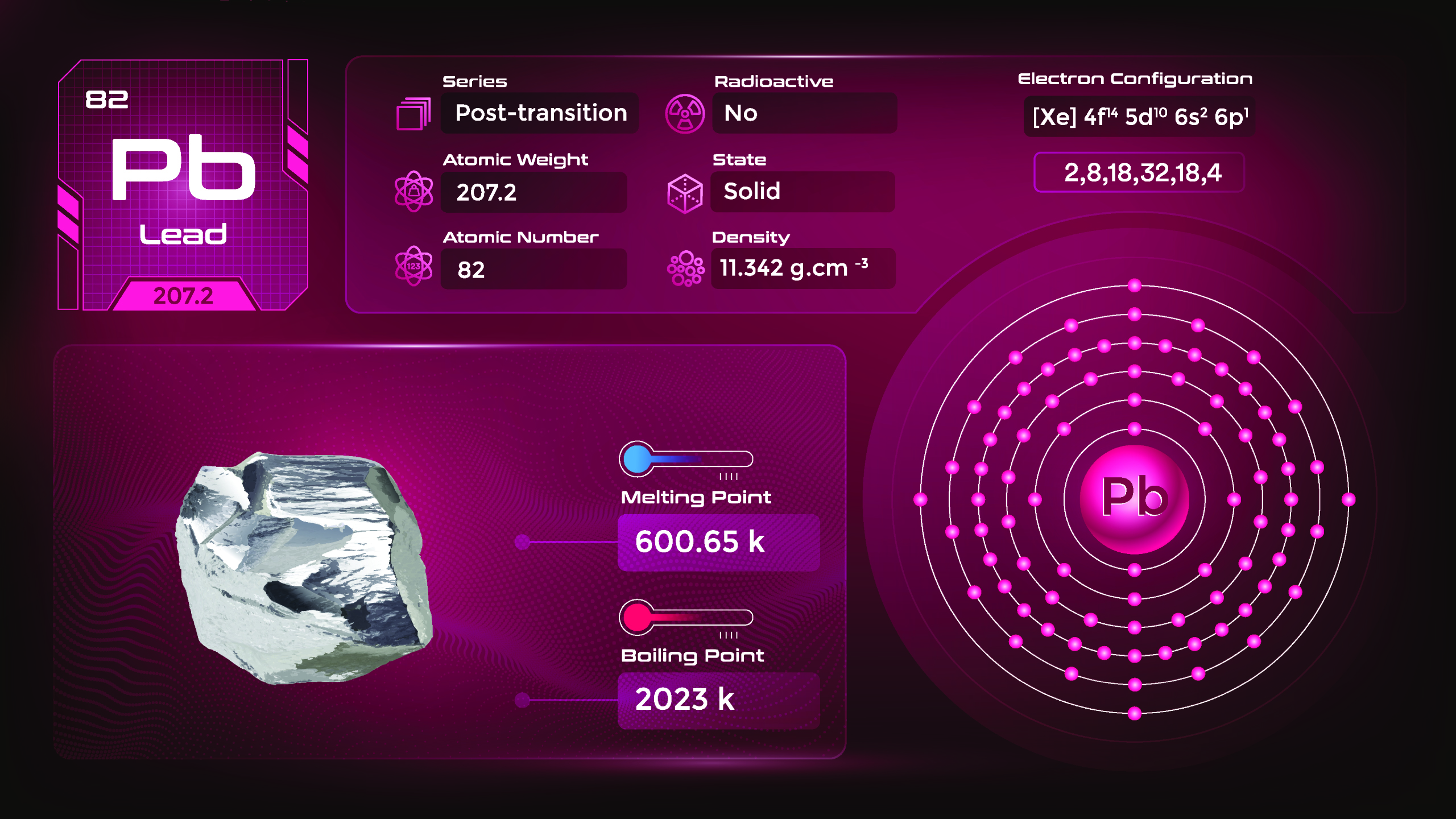
| Name, Symbol | Lead, Pb |
| Atomic number | 82 |
| standard atomic mass | 206.14 |
Lead is a soft, heavy metal with a bluish-white color when freshly cut. It has a low melting point, making it easy to shape and work with. Due to its toxicity, the use of lead in various products has been restricted or banned in many countries. However, lead can react with certain acids and some other chemical compounds. Recycling lead helps reduce environmental contamination and the need for mining new lead ores.
36. Thallium (Tl)
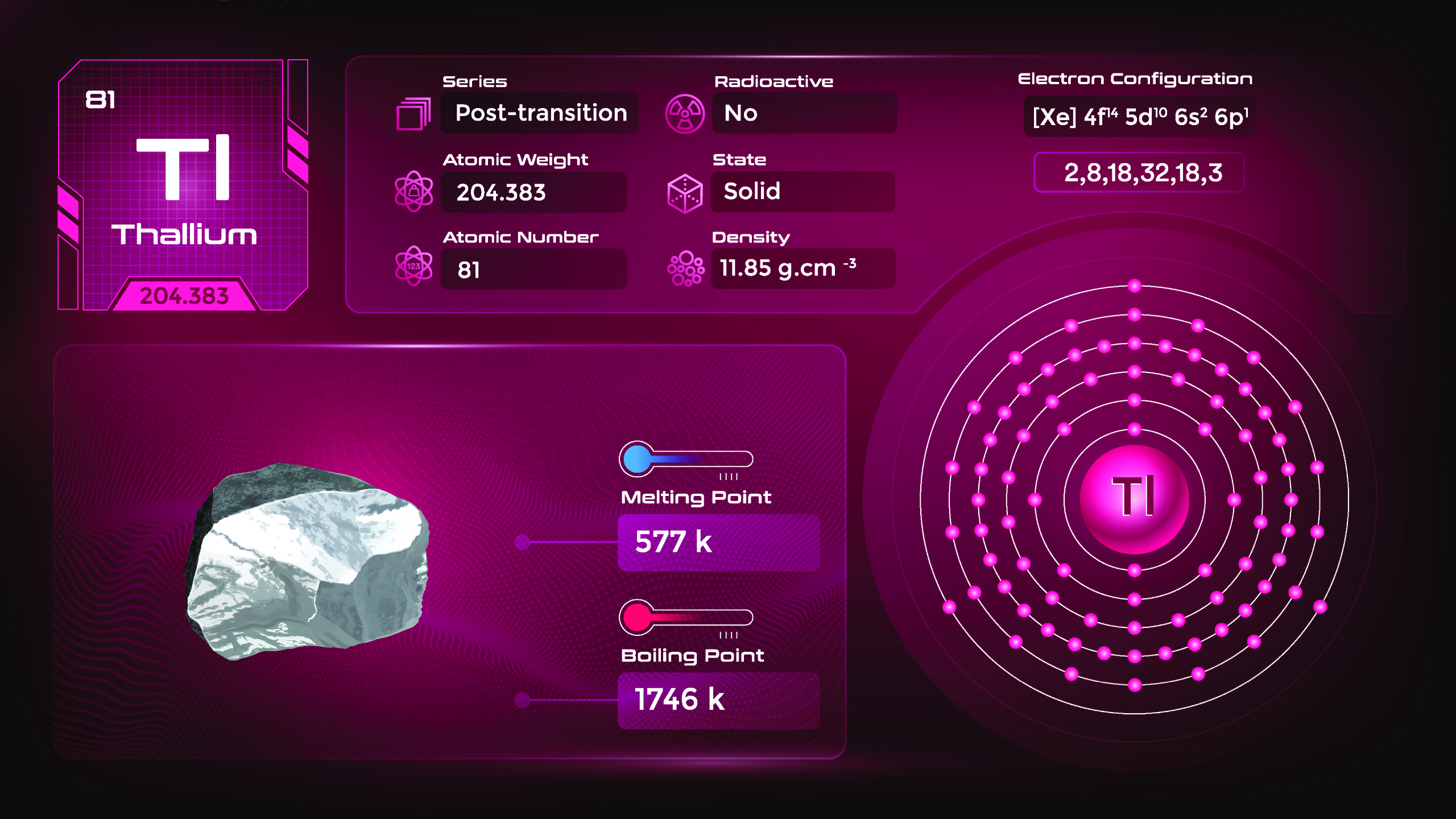
| Name, Symbol | Thallium, Tl |
| Atomic number | 81 |
| standard atomic mass | 204,38 |
Thallium is a bluish-gray metal with a low melting point and low density. It is soft and malleable, meaning it can be easily shaped or flattened with pressure. Thallium and its compounds are highly toxic to living organisms, including humans. Ingesting or inhaling thallium can have severe health effects, affecting the nervous system, kidneys, and other organs. Thallium-201 is a radioactive isotope used in medical imaging, specifically in single-photon emission computed tomography (SPECT) scans.
37. Mercury (Hg)

| Name, Symbol | Mercury, Hg |
| Atomic number | 80 |
| standard atomic mass | 200.6 |
Mercury is the only metal that is liquid at room temperature, giving it a unique appearance. It can enter the body through inhalation, ingestion, or absorption through the skin. However, the use of mercury has been significantly reduced or eliminated in many of these applications due to its toxicity and environmental concerns. When released into the environment, mercury can transform into methylmercury, a highly toxic form that can accumulate in fish and other aquatic organisms, posing risks to human health through consumption.
38. Gold (Au)

| Name, Symbol | Gold, Au |
| Atomic number | 79 |
| standard atomic mass | 196.97 |
Gold is a dense, soft metal with a bright, yellow color. It has a high luster and is highly malleable and ductile, meaning it can be hammered into thin sheets (gold leaf) and drawn into thin wires. Gold is often recovered through mining operations, including open-pit or underground mining. Responsible mining practices aim to mitigate these impacts and minimize harm to ecosystems.
39. Platinum (Pt)

| Name, Symbol | Platinum, Pt |
| Atomic number | 78 |
| standard atomic mass | 195.01 |
Platinum is a silvery-white metal with a bright and lustrous appearance. This corrosion resistance is one of the key reasons why platinum is widely used in various industries. Platinum is relatively rare in the Earth’s crust. It is primarily obtained as a byproduct of nickel and copper mining, as well as through primary platinum mining operations in a few locations globally. Due to its rarity and high demand, platinum is considered a precious metal.
40. Iridium (Ir)

| Name, Symbol | Iridium, Ir |
| Atomic number | 77 |
| standard atomic mass | 192.22 |
Iridium is a silvery-white metal with a very high melting point, making it one of the most heat-resistant elements. It is one of the densest naturally occurring elements and has a high hardness. It is used in spark plugs, electrical contacts, and other devices that require reliable electrical performance. Mining and extracting iridium are complex processes due to its rarity and its occurrence in low concentrations.
41. Osmium (Os)

| Name, Symbol | Osmium, Os |
| Atomic number | 76 |
| standard atomic mass | 190.23 |
Osmium is a bluish-white metal with a high melting point, making it one of the most heat-resistant elements. It is one of the densest naturally occurring elements, surpassed only by iridium. They are used in the production of certain organic compounds, as well as in asymmetric synthesis and oxidation processes. Osmium is also used in certain specialized applications, including fountain pen tips, instrument pivots, and phonograph needles. Due to its rarity and unique properties, mining and extracting osmium are complex processes.
42. Rhenium (Re)
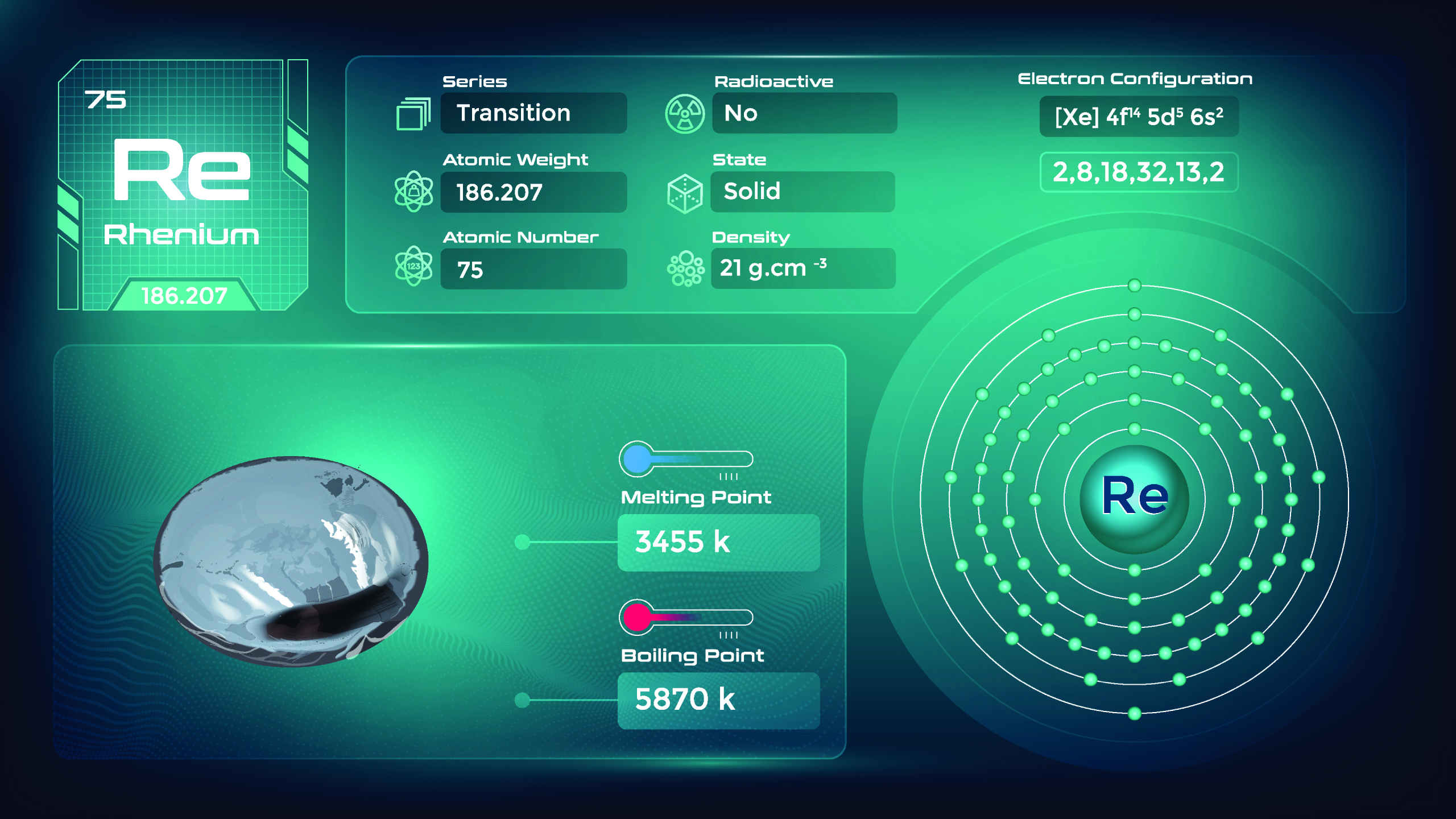
| Name, Symbol | Rhenium, Re |
| Atomic number | 75 |
| standard atomic mass | 186.21 |
Rhenium is a silvery-white, dense transition metal known for its high melting point, remarkable resistance to heat and wear, and unique properties. It is used in the production of certain organic compounds, as well as in the petroleum refining industry and catalytic reforming processes. Its limited availability and high cost contribute to its specific niche applications.
43. Tungsten (W)

| Name, Symbol | Tungsten, or wolfram, W |
| Atomic number | 74 |
| standard atomic mass | 183.84 |
Tungsten is a silver-gray metal with a high melting point, surpassed only by carbon. These properties make tungsten ideal for use in tools, cutting edges, and high-stress applications. It is commonly used in electrical and electronic devices such as light bulb filaments, electron emitters, and electrical contacts in switches and relays. It is used in medical and industrial applications where protection against radiation is necessary, such as in X-ray machines and nuclear power plants.
44. Tantalum (Ta)
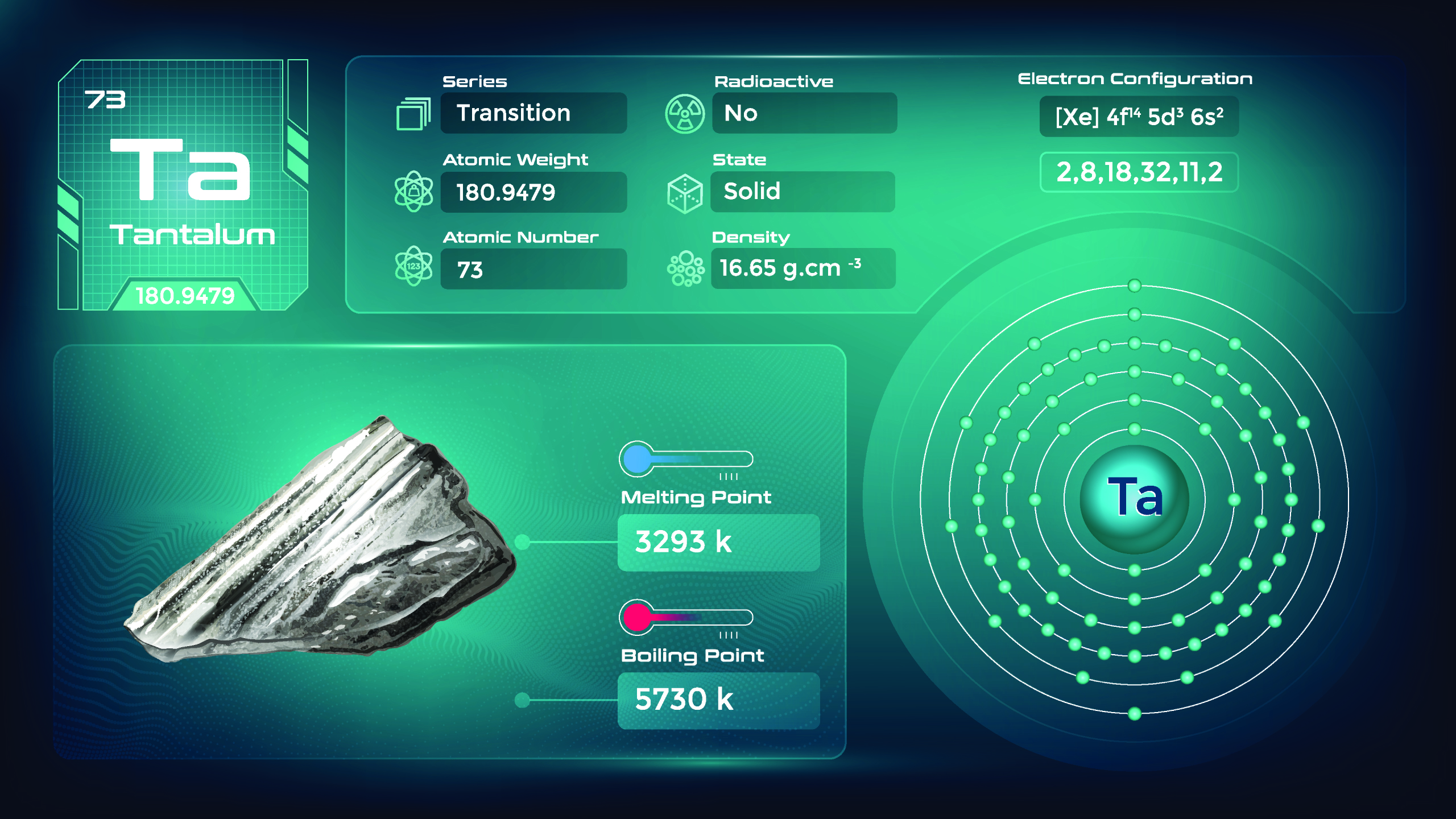
| Name, Symbol | Tantalum, Ta |
| Atomic number | 73 |
| standard atomic mass | 180.95 |
Tantalum is a grayish-blue metal with a high melting point. It is one of the densest elements, surpassed only by a few other metals. Tantalum is highly ductile, meaning it can be drawn into thin wires. Tantalum is a good conductor of electricity. It is used in various electrical and electronic applications, including capacitors. Its availability can be influenced by geopolitical factors, leading to supply chain challenges. Its unique combination of properties and its limited supply contribute to its specific niche uses and value.
45. Hafnium (Hf)

| Name, Symbol | Hafnium, Hf |
| Atomic number | 72 |
| standard atomic mass | 178.49 |
Hafnium is a shiny, silvery-gray metal with a high melting point. It is ductile and malleable, meaning it can be drawn into wires and hammered into thin sheets. They find applications in aerospace components, gas turbines, and chemical processing equipment. Hafnium oxide is used as a high-k dielectric material in the manufacture of advanced semiconductor devices, including capacitors and high-speed transistors. Its production is closely linked to the mining and processing of zirconium ores.
